原子力显微镜
AFM配件
应用
联系我们
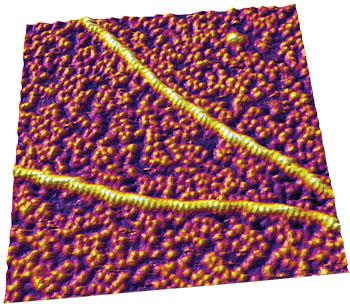
原子力显微镜(AFM)是一种功能强大的仪器,它能够在近生理条件下解析分子结构。样品可以在其原本状态下进行成像:有充分的水分和适宜生物的温度,样品无需额外的制备流程(如固定、喷涂和脱水等)。Asylum Research 原子力显微镜的一个主要优势在于它能够监测动态的行为。由于对样品的处理更大程度地最小化,分子间的相互作用和分子对外界因素的反应是可以观察到的。AFM的另一个功能是测量分子的机械特性,它可以测到低至皮牛(10-12 牛顿力)的数量级,并能够探测到分子内部和分子之间的作用力。通过这些测量,科研人员能够更深入地了解蛋白质动力学,例如:蛋白质是如何自组装的,以及分解它们所需的力。
咨询AFM领域的专家"Multifrequency reveals lipid membrane mechanical properties and the of cholesterol in modulating viscoelasticity," Z. Al-Rekabi and S. Contera, Proc. Natl. Acad. Sci. U.S.A. 115, 2658 (2018). https://doi.org/10.1073/.1719065115
"-resolution structure of DNA G-wires in aqueous solution," K. Bose, C. J. Lech, B. Heddi, and A. T. Phan, Nat. Commun. 9, 1959 (2018). https://doi.org/10.1038/s41467-018-04016-y
"Controlling the mechanoelasticity of model biomembranes with room- ionic liquids," C. Rotella, P. Kumari, B. J. Rodriguez, S. P. Jarvis, and A. Benedetto, Biophys. Rev. 10, 751 (2018). https://doi.org/10.1007/s12551-018-0424-5
"A novel pathway for amyloids self-assembly in aggregates at nanomolar concentration mediated by the interaction with surfaces," S. Banerjee, M. Hashemi, Z. Lv, S. Maity, J. C. Rochet, and Y. L. Lyubchenko, Sci. Rep. 7, 45592 (2017). https://doi/org/10.1038/srep45592
"Endothelial glycocalyx-mediated nitric oxide production in response to selective pulling," A. M. W. Bartosch, R. Mathews, and J. M. Tarbell, Biophys. J. 113, 101 (2017). https://doi.org/10.1016/j.bpj.2017.05.033
"DNA nanostructures-mediated molecular imprinting lithography," C. Tian, H. Kim, W. Sun, Y. Kim, P. Yin, and H. Liu, ACS Nano 11, 227 (2017). https://doi.org/10.1021/acsnano.6b04777
"Self-organized architectures from assorted DNA-framed nanoparticles," W. Liu, J. Halverson, Y. Tian, A. V. Tkachenko, and O. Gang, Nat. Chem. 8, 867 (2016). https://doi.org/10.1038/nchem.2540
"TRF2-mediated control of telomere DNA topology as a mechanism for chromosome-end protection," D. Benarroch-Popivker, S. Pisano, A. Mendez-Bermudez, L. Lototska, P. Kaur, S. Bauwens, N. Djerbi, C. M. Latrick, V. Fraisier, B. Pei, A. Gay, E. Jaune, K. Foucher, J. Cherfils-Vicini, E. Aeby, S. Miron, A. Londoño-Vallejo, J. Ye, M.-H. Le Du, H. Wang, E. Gilson, and M.-J. Giraud-Panis, Mol. Cell 61, 274 (2016). http://dx.doi.org/10.1016/j.molcel.2015.12.009
"Visualizing the path of DNA through proteins using DREEM imaging," D. Wu, P. Kaur, Z. M. Li, K. C. Bradford, H. Wang, and D. A. Erie, Mol. Cell 61, 315 (2016). https://doi.org/10.1016/j.molcel.2015.12.012
"Titin domains progressively unfolded by force are homogenously distributed along the molecule," P. Bianco, Z. Mártonfalvi, K. Naftz, D. Koszegi, and M. Kellermayer, Biophys. J. 109, 340 (2015). https://doi.org/10.1016/j.bpj.2015.06.002
"Direct observation of the reversible two‐state unfolding and refolding of an α/β protein by single‐molecule atomic force microscopy," C. He, C. Hu, X. Hu, X. Hu, A. Xiao, T. T. Perkins, and H. Li, Angew. Chem. Intl. Ed. 54, 9921 (2015). https://doi.org/10.1002/anie.201502938
" of the interaction of the amyloid β (1–42) peptide with short single-stranded synthetic nucleotide sequences: Morphological characterization of the inhibition of fibrils formation and fibrils disassembly," J. N. Abraham, D. Kedracki, E. Prado, C. Gourmel, P. Maroni, and C. Nardin, Biomacromolecules 15, 3253 (2014). https://doi.org/10.1021/bm501004q
"Multiparametric -resolution imaging of native proteins by force-distance curve–based ," M. Pfreundschuh, D. Martinez-Martin, E. Mulvihill, S. Wegmann, and D. J. Muller, Nat. Protoc. 9, 1113 (2014). https://doi.org/10.1038/nprot.2014.070
"The nanomechanical properties of lipid membranes are significantly influenced by the presence of ethanol," F. W. S. Stetter and T. Hugel, Biophys. J. 104, 1049 (2013). https://doi.org/10.1016/j.bpj.2013.01.021
"Non-DLVO adhesion of F-specific RNA bacteriophages to abiotic surfaces: Importance of surface roughness, hydrophobic and electrostatic interactions," C. Dika, M. Ly-Chatain, G. Francius, J. Duval, and C. Gantzer, Colloids Surf. A 435, 178 (2013). https://doi.org/10.1016/j.colsurfa.2013.02.045
"Distinct annular oligomers captured along the assembly and disassembly pathways of transthyretin amyloid protofibrils," R. H. Pires, Á. Karsai, M. J. Saraiva, A. M. Damas, and M. S. Z. Kellermayer, PLoS One 7, e44992 (2012). https://doi.org/10.1371/journal.pone.0044992
"Surface characterization and imaging of mixed fibrinogen–surfactant films," N. Hassan, J. Maldonado-Valderrama, A. P. Gunning, V. J. Morris, and J. M. Ruso, J. Phys. Chem. B 115, 6304 (2011). https://doi.org/10.1021/jp200835j
"Site-specific attachment of proteins onto a 3D DNA tetrahedron through backbone-modified phosphorothioate DNA," N. Y. Wong, C. Zhang, L. H. Tan, and Y. Lu, Small 7, 1427 (2011). https://doi.org/10.1002/smll.201100140
"Tuning the elastic modulus of hydrated collagen fibrils," C. A. Grant, D. J. Brockwell, S. E. Radford, and N. H. Thomson, Biophys. J. 97, 2985 (2009). https://doi.org/10.1016/j.bpj.2009.09.010
"Stepwise dynamics of epitaxially growing single amyloid fibrils," M. S. Z. Kellermayer, A. Karsai, M. Benke, K. Soos, and B. Penke, Proc. Natl. Acad. Sci. U.S.A. 105, 141 (2007). https://doi.org/10.1073/.0704305105
"Packing density and structural heterogeneity of insulin amyloid fibrils measured by nanoindentation," S. Guo, and B. B. Akhremitchev, Biomacromolecules 7, 1630 (2006). https://doi.org/10.1021/bm0600724
" softening of a protein in single-molecule experiments," M. Schlierf and M. Rief, J. Mol. Biol. 354, 497 (2005). https://doi.org/10.1016/j.jmb.2005.09.070
"Reversible mechanical unzipping of amyloid β-fibrils," M. S. Z. Kellermayer, L. Grama, Á. Karsai, A. Nagy, A. Kahn, Z. L. Datki, and B. Penke, J. Biol. Chem. 280, 8464 (2004). https://doi.org/10.1074/jbc.m411556200
"Segmented nanofibers of spider dragline silk: Atomic force microscopy and single-molecule force ," E. Oroudjev, J. Soares, S. Arcidiacono, J. B. Thompson, S. A. Fossey, and H. G. Hansma, Proc. Natl. Acad. Sci. U.S.A. 99, 6460 (2002). https://doi.org/10.1073/.082526499
"Can non-mechanical proteins withstand force? Stretching barnase by atomic force microscopy and molecular dynamics simulation," R. B. Best, B. Li, A. Steward, V. Daggett, and J. Clarke, Biophys. J. 81, 2344 (2001). https://doi.org/10.1016/s0006-3495(01)75881-x
 公安机关备案号31010402003473
公安机关备案号31010402003473